Which of the Following Describes a Synthesis Reaction
Use the worksheet and quiz to practice the following skills. In all reactions electrons are passed from the reduced form of one reactant a lower reduction potential to the oxidized form of the next reactant a high reduction potential.
Chemistry Module 5 Types Of Chemical Reactions Http Www Youtube Com Watch V Aawccqb75d0 Single
Click again to see term.

. ATP is converted to ADP. Select all apply Points will be deducted for incorrect answers Reaction of a tertiary alcohol with chromic acid Reaction of 1-methylcyclohexene with Oz followed by CH32S Hydration of terminal alkyne Hydroboration-Oxidation of terminal alkyne Reaction of an ester with DIBAL-H followed. In a chemical reaction dynamic equilibrium occurs when the rate of the forward reaction becomes precisely equal to the rate of the reverse reaction.
Energy of activation E. Which of the following describes the function of the pigment molecules in a light-harvesting complex in the thylakoid membranes. Two amino acids are bonded together to form a dipeptide.
Sucrose is chemically separated to form one molecule of glucose and one molecule of fructose. 05112019 Chemistry Secondary School answered Which of the following reactions is a synthesis reaction. 2Mg O2 ---- 2MgO.
The nucleotide chains of DNA are held together by _____. Which of the following is a synthesis reaction. Decomposition reactions are essentially the opposite of synthesis reactions 2H2O 2K s 2KOH l 2H2 g is an example of which type of reaction.
Answer 23 5 3 Brainly User Answer. Click card to see definition. The standard form of a synthesis reaction is A B AB.
B They absorb and transfer light energy to the reaction-center chlorophyll. Which of the following methods describes a synthesis of an aldehyde. In a synthesis reaction two or more elements or compounds combine to synthesize or create a new compound.
KOH HNO3 HOH KNO3 Synthesis Decomposition Single Replacement. Which of the following represents the balanced chemical equation for the Synthesis reaction that forms magnesium oxide MgO. All of the following statements are true EXCEPT A.
Which of the following terms describes ATP synthesis in mitochondria. A They split water and release oxygen from the reaction-center chlorophyll. What type of reaction is this.
Atoms or molecules combine Energy is absorbed for bond formation. An increase in which of the following results in a decrease in the rate of a reaction. Single-displacement When cadmium Cd is mixed with hydrochloric acid HCl a single-displacement reaction occurs.
Critical thinking - apply relevant concepts to examine information about dehydration synthesis reactions in a different light. What will be the product or products. Add your answer and earn points.
Because energy is absorbed to make bonds synthesis reactions are energy absorbing reactions. Two smaller molecules join together after a water molecule is removed from between them. A treatment of an alkene with Sia 2 BH B hydrogenation of an acid chloride using PdBaSO S as a poisoned catalyst 32 4 C reaction of a primary alcohol with Na 2 Cr 2 O 7 D reaction of a ketone with ozone E none of the above SHORT ANSWER.
N 2 3H 2 2NH 3 MgI 2 Br 2 MgBr 2 I 2 KClO3 KCl O2 HCl NaOH NaCl H 2 O 1 See answer Add answer 5 pts Advertisement kpchild04 is waiting for your help. C They synthesize ATP from ADP and Pi. One product and two reactants.
Molecule is broken down. Decomposition reaction ABA B synthesis reactions is reverse. A chemical reaction can be defined as a chemical process involving the continuous transformation and rearrangement of either the ionic atomic or molecular structure of a chemical element especially through the bre.
Sodium chloride is dissolved in water. Putting together into one. 32 Which of the following describes a synthesis of an aldehyde.
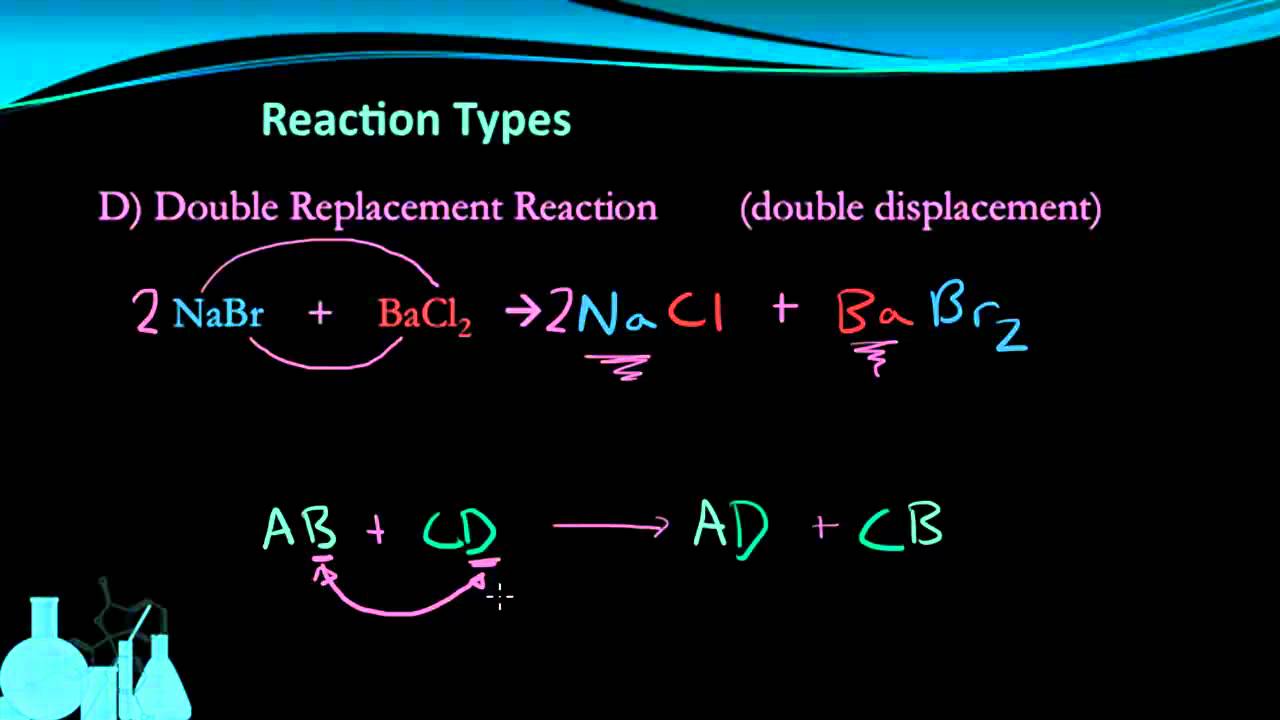
Tap card to see definition. The definition of synthesis in chemistry is the production of chemical compounds by reaction from simpler materials A is decomposition B is DOUBLE replacement and C is SINGLE replacement. Which of the following describes the reaction shown below.
4H O2 ---- 2H2O. Which of the following best describes dehydration synthesis. Tap again to see term.
The phrase which best describes a synthesis reaction is. Click again to see term.

A Synthesis Reaction Is A Type Of Reaction In Which Multiple Reactants Combine To Form A Single Product Synthesis React Chemical Reactions Chemistry Synthesis

Decomposition Reactions Youtube Chemical Reactions Chemical Equation Reactions

What Is A Synthesis Reaction Definition And Examples Synthesis Reactions Reaction Types
Comments
Post a Comment