Describe the Dissolving Process at the Molecular Level
Eventually the particle detaches from the remaining solute surrounded by solvent molecules in solution. Up to 24 cash back There are 3 steps to the dissolving process.

4 1c Solution Chemistry Describe The Dissolving Process Cset Study Guide Chemistry
Recall the rule that like dissolves like.

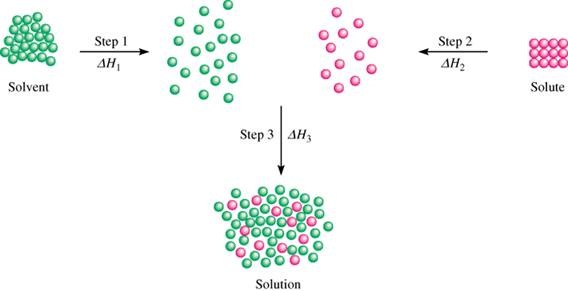
. Dissolving a solid in a liquid depends on the interactions and attractions between the molecules of the liquid solvent and the particles of the solid solute. Dissolving happens when the attractions between the water molecules and the sodium and chloride ions overcome the attractions of the ions to each other. As illustrated in Figure 3 the formation of a solution may be viewed as a stepwise process in which energy is consumed to overcome solute-solute and solvent-solvent attractions endothermic processes and released when solute-solvent attractions are established an exothermic process referred to as solvation.
To understand this process at the molecular level we must apply the three steps we previously discussed. At the molecular level the individual solid molecules become separated and interact less with each other and more with surrounding solvent molecules. What occurs at the molecular level to cause a solute to dissolve in a solvent.
As illustrated in Figure 3 the formation of a solution may be viewed as a stepwise process in which energy is consumed to overcome solute-solute and solvent-solvent attractions endothermic processes and released when solute-solvent attractions are established an exothermic process referred to as solvation. Use the dissolution of a solid in a liquid as an example. Recall the rule that like dissolves like.
This means that substances must have similar intermolecular forces to form solutions. This nonstop attack continuous until the whole NaCl crystal disintegrates. Sodium chloride NaCl dissolves when water molecules continuously attack the NaCl crystal pulling away the individual sodium Na and chloride Cl ions.
The relative magnitudes of the energy changes. In molecular nature similar solute molecules are. Use the dissolution of a solid in a liquid as an example.
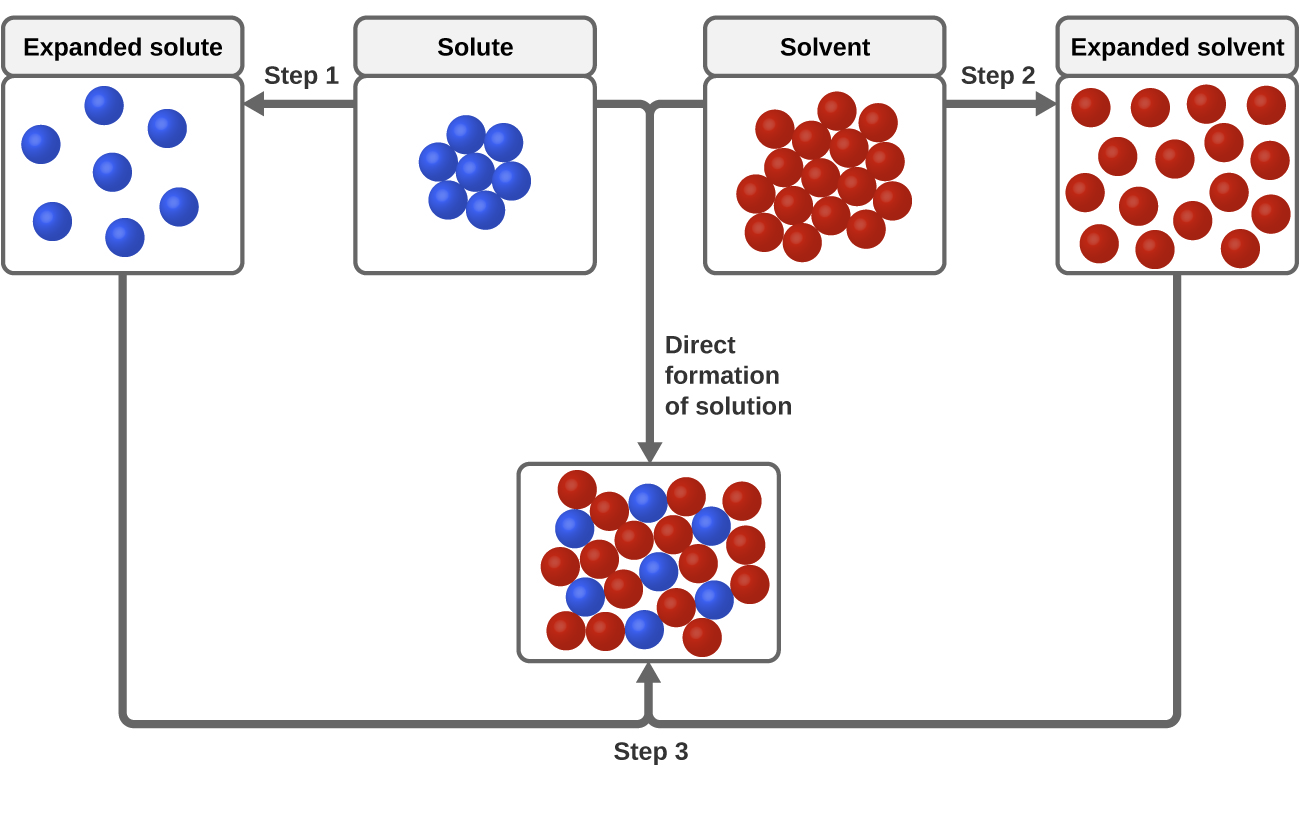
The solute particles must separate form the other solute particles. The copper then physically flows to the item where it is reduced to the metallic state by gaining. Briefly describe the solution process at the molecular level.
The answer depends in part on the solute but there are some similarities common to all solutes. Chemistry 10th Edition Edit edition. At the molecular level the individual solid molecules become separated and interact less with each other and more with surrounding solvent molecules.
Describe the process of dissolving. The concept of dissolving is when a solid called a solute completely mixes with a liquid called a solvent at the molecular level to become a single visible phase and become a homogeneous. The concept of dissolving is when a solid called a solute completely mixes with a liquid called a solvent at the molecular level to become a single visible phase and become a homogeneous mixture.
This causes the ions to separate from one another and become thoroughly mixed into the water. Describe on a molecular level the difference between the two physical changes melting and dissolving Step-by-step solution Chapter 33 Problem 3E is solved. The solvent particles must move apart to make room for solute particles.
This problem has been solved. This takes place at the surface of the solute. The answer depends in part on the solute but there are some similarities common to all solutes.
Briefly describe the solution process at the molecular level. The first step in the dissolving process is endothermic. Describe what happens on the molecular level as the water dissolves in the isopropanol.
Students look at illustrations and animations of these substances on the molecular level and see that the process of dissolving involves the attraction and interaction of the molecules of the liquid and the solid. Problem 3 Easy Difficulty. When a soluble solute is introduced into a.
Dissolving needs another term to be used this is solvation. In the case of molecular solutes like glucose the solute particles are individual molecules. So a solvent film occurs around the molecule in other wordsa solvent-like micro-environment is formed.
This process requires energy to overcome forces of attraction between solvent particles. Dissolving implies that the solute and solvent interact to create a solution. What occurs at the molecular level to cause a solute to dissolve in a solvent.
Dissolving occurs when the solute is pulled apart by the solvent. The polar isopropanol molecules are attracted to the. The dissolving process is how a solution is formed.
When a solute dissolves the individual particles of solute become surrounded by solvent particles. The concept of dissolving is when a solid called a solute completely mixes with a liquid called a solvent at the molecular level to become a single visible phase and become a homogeneous mixture. As the solute dissolves in the solvent the particles of the solute begin.
The relative magnitudes of the energy changes. The answer depends in part on the solute but there are some similarities common to all solutes. Solvation is briefly surrounding of solvent molecules around solute molucule.
When a soluble solute is introduced into a. Solutions for Chapter 12 Problem 3RQ. The Dissolution Process.
This helps the solvated molecule be soluble in the solvent. Students see that a liquids ability to dissolve a substance is a characteristic property that can be used to identify the liquid. Dissolving happens when the attraction between the particles of the solvent and solute are strong enough to overcome the attraction of the particles of the solute for one another.
Copper plating is the process of plating a layer of copper electrolytically on the surface of an item. Recall the rule that like dissolves like. What occurs at the molecular level to cause a solute to dissolve in a solvent.
As we saw in Section 91 Solutions this means that substances must have similar intermolecular forces to form solutions. As we saw in Section 91 Solutions this means that substances must have similar intermolecular forces to form solutions. As the copper ions dissolve into the water they form a coordination complex with salts already present.
Dissolving Process Ms Jonasson S Classes
Comments
Post a Comment